Your Location:Home > Products > API > Agomelatine
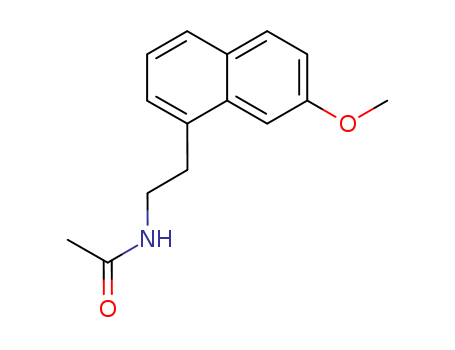

CasNo: 138112-76-2
MF: C15H17NO2
Appearance: white solid
|
Description |
Agomelatine is an atypical antidepressant used primarily to treat major depressive disorder and generalized anxiety disorder. It operates uniquely as both a melatonin receptor agonist (MT1 and MT2) and a serotonin 2C (5-HT2C) receptor antagonist. This dual action helps regulate circadian rhythms and improve sleep quality, which are often disrupted in depressive patients, without the typical side effects associated with SSRIs and SNRIs. |
|
Uses |
Agomelatine, developed by the French company Servier, is more metabolically stable than melatonin due to its naphthalene structure replacing the indole ring of melatonin. Its efficacy stems from its ability to stabilize circadian rhythms and exert antidepressant effects through these receptors, leading to improvements in both mood and sleep. Despite its benefits, agomelatine is associated with risks of liver toxicity, necessitating regular liver function tests. The drug is metabolized primarily by the hepatic cytochrome P450 enzyme CYP1A2, with minor contributions from CYP2C9 and CYP2C19, and its metabolites are inactive and excreted in the urine. |
InChI:InChI=1/C11H14N4O2.CH4O3S/c1-5-2-9(16)7-3-8(14-15-11(12)13)10(17)4-6(5)7;1-5(2,3)4/h3-5,9,16H,2H2,1H3,(H4,12,13,15);1H3,(H,2,3,4)/b14-8-;
A simple and efficient process for the l...
Agomelatine, the first melatonergic antidepressant, was designed to improve depressed states by resynchronizing perturbed biological rhythms. Its 'synergistic' agonist properties at melatonin receptors plus antagonist properties at 5-hydroxytryptamine 2C (5-HT2C) receptors account for its beneficial influence on depressed states.
Agomelatine was significantly more effective than placebo with an effect size (SMD) of 0.24 (95% confidence interval 0.12 to 0.35) and relative risk of response 1.25 (1.11 to 1.4). Compared with other antidepressants, agomelatine showed equal efficacy (SMD 0.00, −0.09 to 0.10). Significant heterogeneity was uncovered in most analyses, though risk of bias was low. Published studies were more likely than unpublished studies to have results that suggested advantages for agomelatine.
A series of N-naphthylethyl amide deriva...
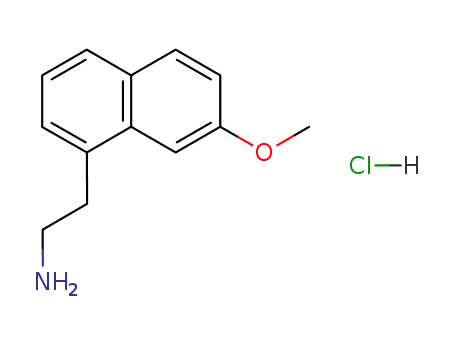
2-(7-methoxynaphthalen-1-yl)ethanamine hydrochloride
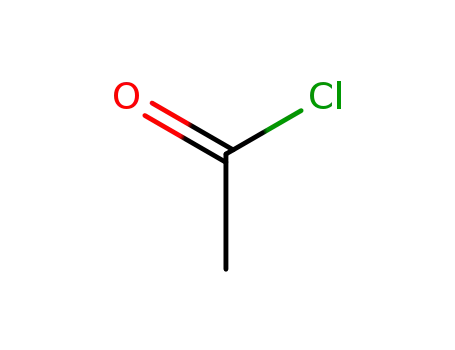
acetyl chloride
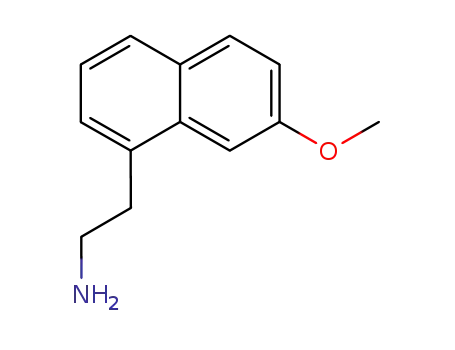
2-(7-methoxynaphth-1-yl)ethylamine
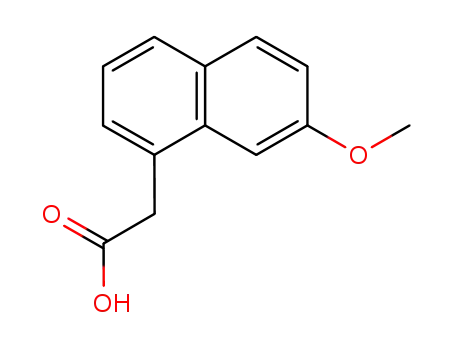
2-(7-methoxynaphthalen-1-yl)acetic acid
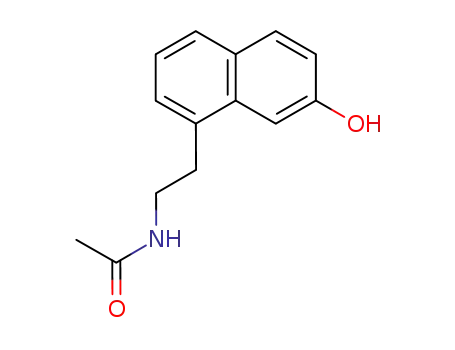
N-[2-(7-Hydroxy-1-naphthyl)ethyl]-acetamide
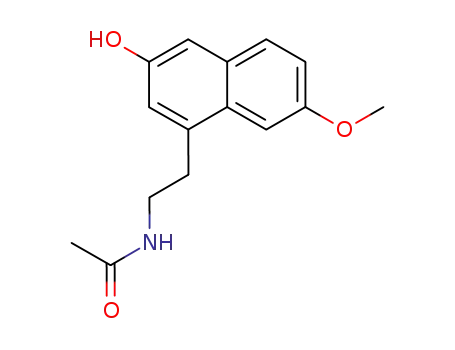
N-[2-(7-methoxy-3-hydroxynaphth-1-yl)ethyl]-acetamide
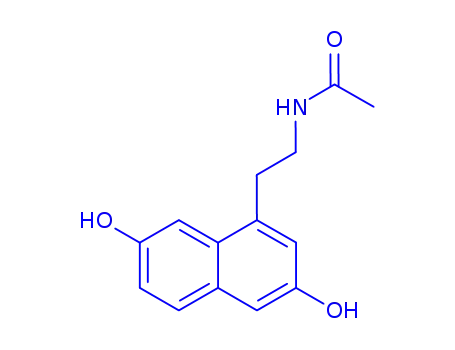
N-[2-(3,7-dihydroxy-1-naphthyl)ethyl]acetamide
N-(2-{7-[(4-methoxybenzyl)amino]naphth-1-yl}ethyl)acetamide