Your Location:Home > Products > Organic Chemistry > Sodium triacetoxyborohydride
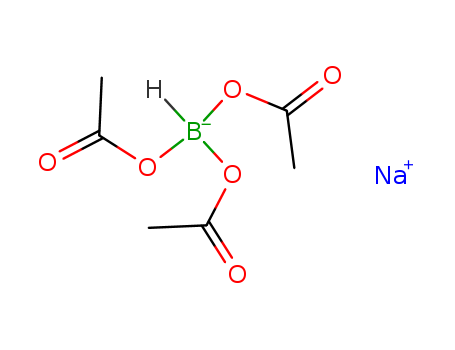

CasNo: 56553-60-7
MF: C6H10BNaO6
Appearance: white crystalline powder
|
Description |
Sodium triacetoxyborohydride (STAB) is a mild, selective, and frequently used reducing agent for reductive amination transformations. |
|
Uses |
Sodium triacetoxyborohydride is a versatile reducing agent widely used in organic synthesis, particularly for reductive amination of aldehydes and ketones to primary, secondary, and tertiary amines. This white crystalline solid is more stable and selective compared to other hydride reagents, making it valuable in complex molecule synthesis. Sodium triacetoxyborohydride is known for its mild reactivity, which allows for selective reductions in the presence of other functional groups without over-reduction. |
InChI:InChI=1/C6H10BO6.Na/c1-11-4(8)7(5(9)12-2)6(10)13-3;/h7H,1-3H3;/q-1;+1/rC6H10BNaO6/c1-12-4(9)7(8,5(10)13-2)6(11)14-3/h7H,1-3H3
Herein we present an overview of the use of sodium triacetoxyborohydride in the reductive amination of ketones and aldehydes, with an emphasis on scope. In general, this is an …
A family of nitroxide biradicals was synthesized for the purpose of creating terminal groups with high relaxivity for linkage to dendrimers for use as targeted MR contrast agents for articular cartilage. The structure of the nitroxide biradicals strongly influenced their MR relaxivities. The addition of a carbon atom between the nitroxyl rings and the bridging nitrogen increased the relaxivity of the nitroxide biradical by ∼14% over what was expected by doubling the relaxivity of the monomeric nitroxide.
The thermal hazards associated with the ...
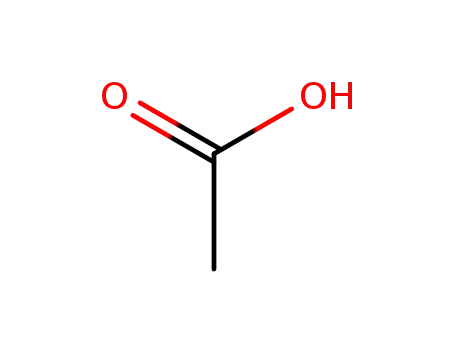
acetic acid

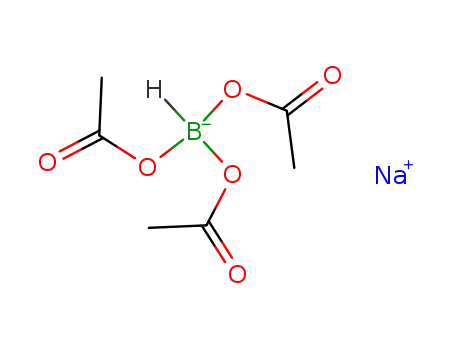
sodium tris(acetoxy)borohydride
| Conditions | Yield |
|---|---|
|
With sodium tetrahydroborate; In benzene;
|
|
|
With sodium tetrahydroborate; at 15 - 20 ℃;
|
|
|
With sodium tetrahydroborate; In 2-methylpropyl acetate; at 0 - 5 ℃; for 1h;
|
|
|
With sodium tetrahydroborate; In dichloromethane; for 2h;
|
|
|
With sodium tetrahydroborate; In tetrahydrofuran; at 0 - 20 ℃; Inert atmosphere; Schlenk technique;
|
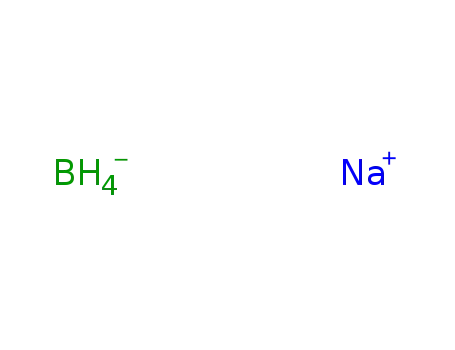
sodium tetrahydroborate


sodium tris(acetoxy)borohydride
| Conditions | Yield |
|---|---|
|
With acetic acid; In benzene; byproducts: H2; under N2-atmosphere; slurry of NaBH4 in benzene cooled (10°C); CH3COOH added dropwise (temp. < 20°C); mixt. warmed (ambient temp.) and stirred for 8h;; filtration; white powder washed (ether) and held under vacuum over night; elem. anal.;;
|
92% |