Your Location:Home > Products > pharmaceutical intermediates > Bentazepam
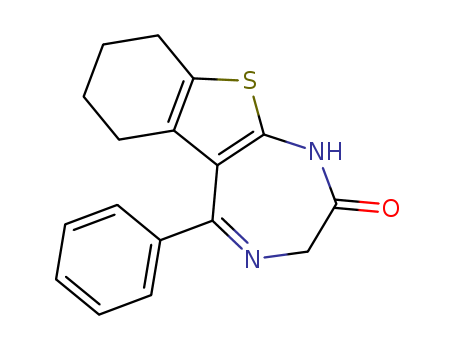

CasNo: 29462-18-8
MF: C17H16 N2 O S
|
Description |
Bentazepam is a thienodiazepine which is a benzodiazepine analog. It possesses anxiolytic, anticonvulsant, sedative and skeletal muscle relaxant properties. Peak plasma rates are achieved in around 2,5 hours after oral administration. The elimination half-life is between approximately 2–4 hours. |
|
Therapeutic Function |
Anticonvulsant, Tranquilizer |
|
Safety Profile |
Moderately toxic by ingestion andintraperitoneal routes. When heated to decomposition itemits toxic fumes of SOx and NOx. |
| Supplier | Dorne chemical technology co. LTD is a comprehensive chemical enterprise integrating r&d, production and sales. It is a leading manufacturer and supplier of chemical products in China.We develop, produce and market high quality pharmaceuticals, intermediates, specialty chemicals and OLED intermediates, as well as other fine chemicals.Relying on the laboratory and taking the factory as the base, the company has the whole process ability of fine chemical product research and development, production and processing, testing and packaging to export. |
InChI:InChI=1/C17H16N2OS/c20-14-10-18-16(11-6-2-1-3-7-11)15-12-8-4-5-9-13(12)21-17(15)19-14/h1-3,6-7,18H,4-5,8-10H2
A 6-week, parallel, randomized, open-label study was conducted to evaluate the effects of bentazepam 75 mg/day versus those of clorazepate 30 mg/day in 50 patients with anxiety disorders. Both drugs reduced anxiety as measured by the Hamilton Anxiety Scale (HAS) and the State-Trait Anxiety Inventory (STAI). The mean HAS and STAI scores at weeks 1, 3, and 6 in patients receiving bentazepam were significantly lower than those of patients receiving clorazepate. Patients taking bentazepam also exhibited improved performance on cognitive tests (Digit-Symbol Substitution Test and Digit Span).
Bentazepam (BTZ), 75 mg/day, and ketazolam (KZ), 30 mg/day, were compared in a parallel, randomized, non-blind study conducted with 40 outpatients diagnosed with anxiety disorders according to the Diagnostic and Statistical Manual of Mental Disorders, revised third edition. After a 1-week washout period to anxiolytic drugs, patients were assigned to either BTZ (n = 20) or KZ (n = 20) treatment. Both the Hamilton Anxiety Rating Scale (HARS) and State subscale from the State-Trait Anxiety Inventory of Spielberger (STAI-S) were administered at baseline and at weeks 1, 3, and 6 of treatment.
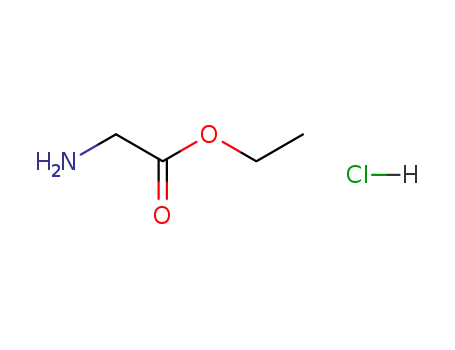
glycine ethyl ester hydrochloride
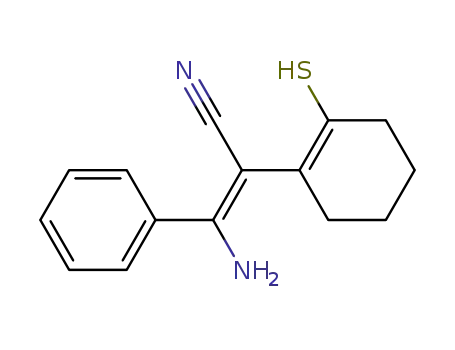
(E)-3-amino-3-phenyl-2-(2-thioxo-cyclohexyl)-acrylonitrile
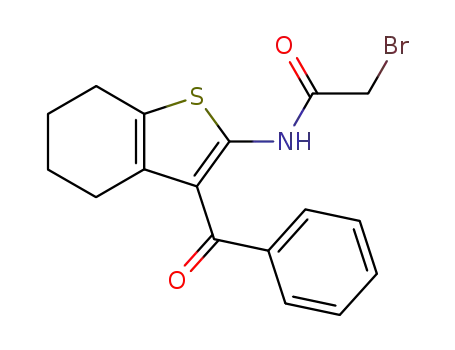
[2-(2-bromo-acetylamino)-4,5,6,7-tetrahydro-benzo[b]thiophen-3-yl]-phenyl-methanone
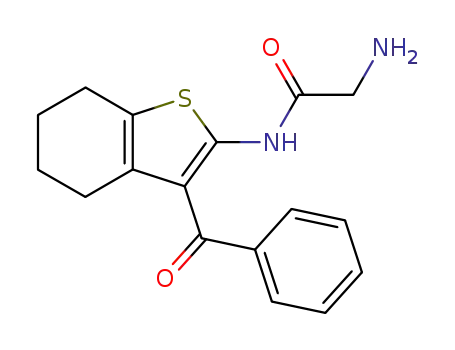
glycine 3-benzoyl-4,5,6,7-tetrahydro-benzo[b]thiophen-2-ylamide
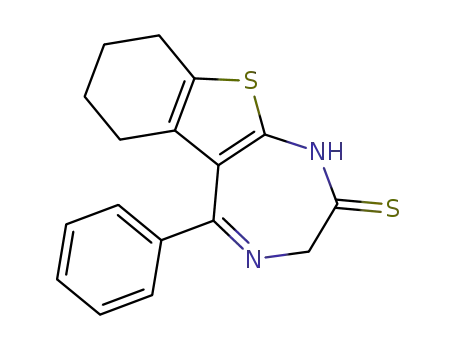
5-phenyl-1,3,6,7,8,9-hexahydro-benzo[4,5]thieno[2,3-e][1,4]diazepine-2-thione
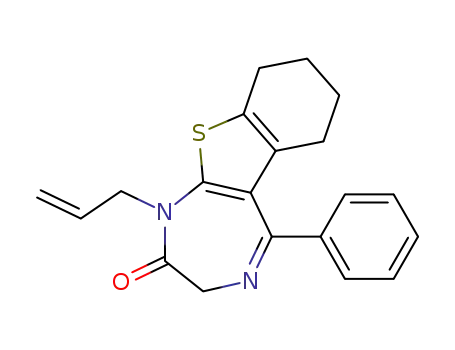
1-allyl-5-phenyl-1,3,6,7,8,9-hexahydro-benzo[4,5]thieno[2,3-e][1,4]diazepin-2-one
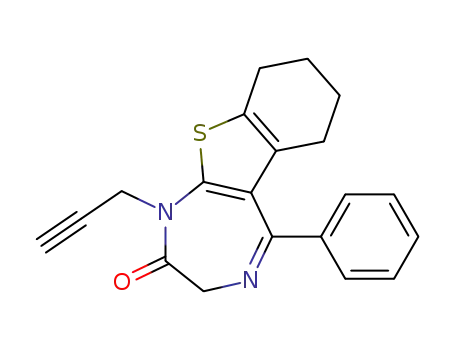
5-phenyl-1-prop-2-ynyl-1,3,6,7,8,9-hexahydro-benzo[4,5]thieno[2,3-e][1,4]diazepin-2-one
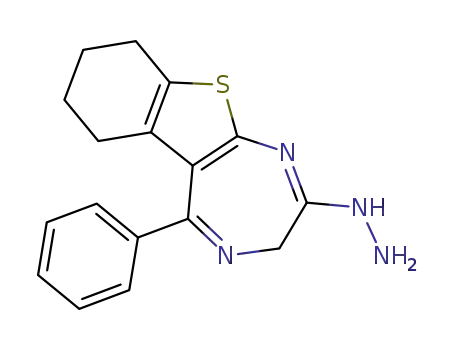
5-phenyl-1,3,6,7,8,9-hexahydro-benzo[4,5]thieno[2,3-e][1,4]diazepin-2-one hydrazone