Your Location:Home > Products > Biochemical Engineering > Medetomidine HCl
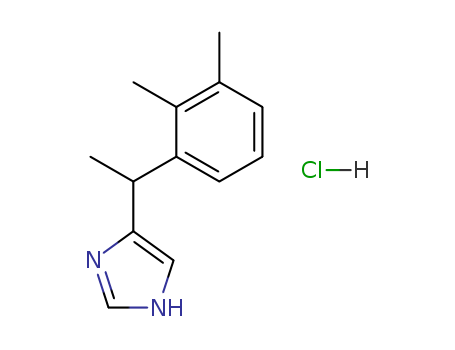

CasNo: 86347-15-1
MF: C13H16N2.HCl
Appearance: White or off white crystalline powder
| Description | Medetomidine hydrochlorideProduct ingredient for Medetomidine. Medetomidine is a synthetic compound used as a surgical anesthetic and analgesic. It is normally found as its hydrochloride salt, medetomidine hydrochloride. Medetomidine is an intravenously available alpha-2 adrenergic agonist. |
|
Biological Activity |
medetomidine is a selective α2-adrenoceptor agonist, with ki of 1.08 nm, exhibts 1620-fold selectivity over α1-adrenoceptor. |
InChI:InChI=1/C13H16N2.ClH/c1-9-5-4-6-12(10(9)2)11(3)13-7-14-8-15-13;/h4-8,11H,1-3H3,(H,14,15);1H
A 2-phase (7-day interval) study was performed. Sedative characteristics (phase I) and cardiopulmonary effects (phase II) of medetomidine hydrochloride and xylazine hydrochloride administration followed by atipamezole hydrochloride administration were evaluated. In both phases, calves were randomly allocated to receive 1 of 4 treatments IV: medetomidine (0.03 mg/kg) followed by atipamezole (0.1 mg/kg; n = 6), xylazine (0.3 mg/kg) followed by atipamezole (0.04 mg/kg; 7), medetomidine (0.03 mg/kg) followed by saline (0.9% NaCl; 6) solution (10 mL), and xylazine (0.3 mg/kg) followed by saline solution (10 mL; 6). Atipamezole or saline solution was administered 20 minutes after the first injection.
To evaluate the cardiovascular effects of the α2-adrenergic receptor agonist medetomidine hydrochloride in clinically normal cats. Heart rate, cardiac index, stroke index, ratepressure product, and right and left ventricular stroke work index significantly decreased from baseline after medetomidine administration, whereas systemic vascular resistance and central venous pressure increased. However, systolic, mean, and diastolic arterial pressures as well as arterial pH, and oxygen and carbon dioxide tensions were not significantly different from baseline values.
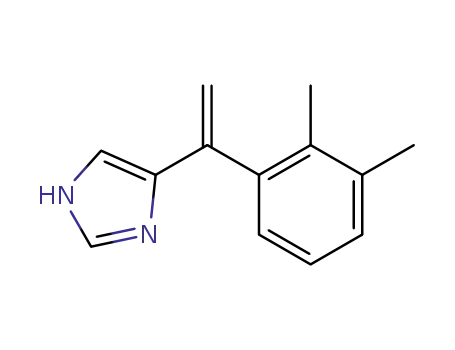
1-(4-imidazolyl)-1-(2,3-dimethylphenyl)ethylene
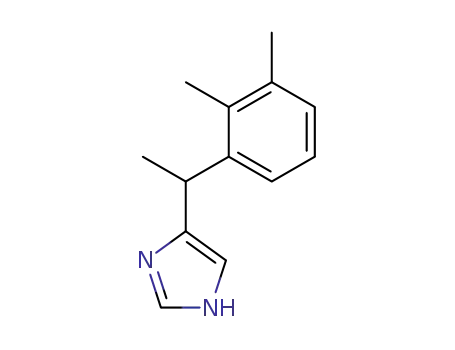
medetomidine
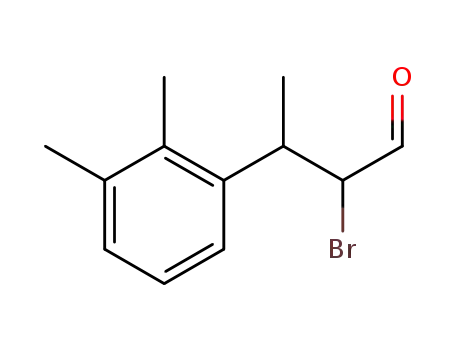
2-bromo-3-(2,3-dimethylphenyl)butanal
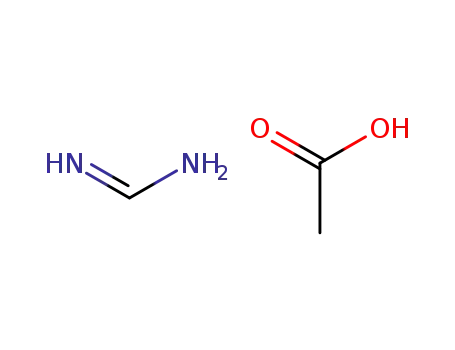
formamidine acetic acid

medetomidine
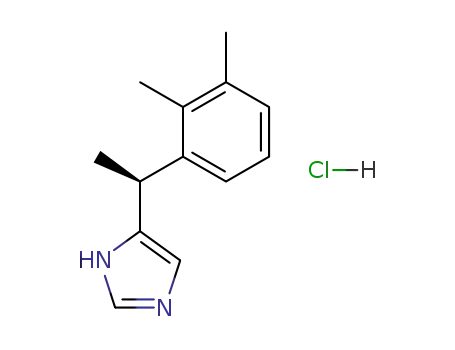
(+)-(S)-4-(1-[2,3-dimethylphenyl]ethyl)-1H-imidazole hydrochloride