Your Location:Home > Products > Inorganic chemistry > Sodium cyanoborohydride
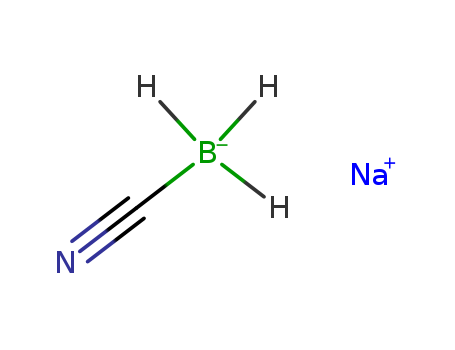

CasNo: 25895-60-7
MF: NaBH3CN
Appearance: white crystalline powder
|
Preparation |
Synthesis of Sodium cyanoborohydride(NaBH3CN): NaBH3CN was made by stirring equimolar BH3·THF (~1 M) with NaCN in THF in excellent yield.To a rapidly stirred slurry of sodium borohydride (80.2g, 2.09 mol) in THF (1000mL) in a 2-L flask is added a solution of hydrogen cyanide in THF (294g containing 58.8g of hydrogen cyanide) at 25°C. Evolution of hydrogen occurs slowly during the addition. Following the addition, the reaction mixture is stirred for 1 h at 25°C and then heated at reflux until hydrogen evolution has ceased. Filtration followed by vacuum removal of THF gives white solid sodium cyanoborohydride; yield 120g (91%). |
|
Reactions |
Sodium cyanoborohydride are frequently used for reductive aminations. Since the reaction rate for the reduction of iminium ions is much faster than for ketones or even aldehydes, the reductive amination can be carried out as a one-pot procedure by introducing the reducing agent into a mixture of the amine and carbonyl compound.Contact with strong acids liberates the highly toxic gas HCN. A safer reducing agent with comparable reactivity is sodium triacetoxyborohydride. Reduction with Sodium Cyanoborohydride:Tin-free Giese reaction of alkyl iodides with electron-deficient alkenes and the related radical carbonylation process proceeded efficiently in the presence of sodium cyanoborohydride and tetrabutylammonium cyanoborohydride. Transfer of iodine followed by hydride reduction of the resulting carbon-iodine bond is proposed as a possible mechanism.Borch and co-workers showed that sodium cyanoborohydride and lithium cyanoborohydride are acid-stable reagents capable of rapidly reducing carbonyl compounds to alcohols at pH 3-4, presumably via a protonated carbonyl cation.With care to maintain a pH of 6-7, a mixture of a ketone or aldehyde reactant, an amine, and sodium cyanohydride provides products of reductive amination selectively, without competitive reduction of the carbonyl substrate. Though the conditions of the Borch reduction are mild, sodium cyanoborohydride is highly toxic, as are its byproducts. The pH was maintained by addition of HCl and/or KOH as needed using bromocresol green as an indicator. |
|
Purification Methods |
Sodium cyanoborohydride (10g) is dissolved in THF (80mL) and 1 M methanolic hydrochloric acid is added until pH reaches 9. The solution is then poured with stirring into dioxan (250mL). The precipitate is collected and stirred for 2 h in ethyl acetate (250mL). This solution is filtered, heated to reflux on a steam bath and then dioxan (150mL) is added slowly with swirling. This solution is slowly cooled to room temperature, chilled and filtered. The crystalline dioxan complex is dried in vacuo for 4 h at room temperature, then for 4 h at 80° C; yield 6.74g, purity >98% sodium cyanoborohydride by iodometric titration. |
InChI:InChI=1/CH3BN.Na/c2-1-3;/h2H3;/q-1;+1
A convenient synthetic procedure for sod...
Methods, compositions and kits are discl...
The present invention relates to cyclopr...
Mono- and di-cyanohydroborate and cyanob...
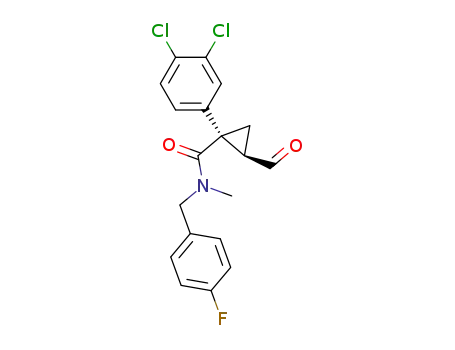
(1S,2R)-1-(3,4-dichlorophenyl)-2-formyl-cyclopropanecarboxylic acid (4-fluorobenzyl)methylamide

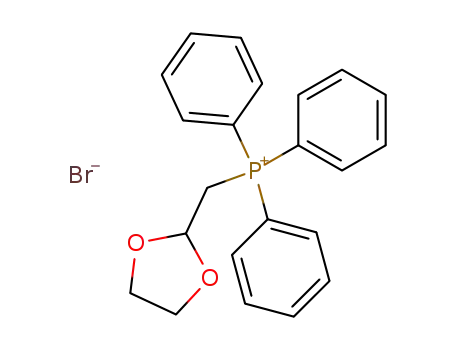
(1,3-dioxolan-2-yl-methyl)triphenylphosphonium bromide

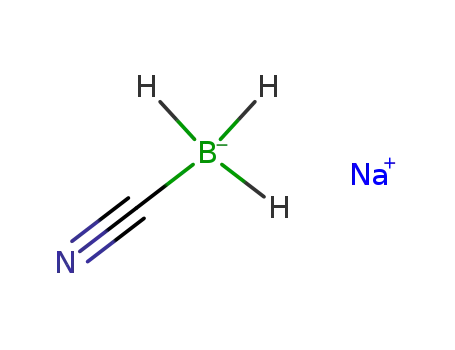
sodium cyanoborohydride
| Conditions | Yield |
|---|---|
|
With potassium hexamethylsilazane; In tetrahydrofuran; at 20 ℃; for 2h;
|
91% |
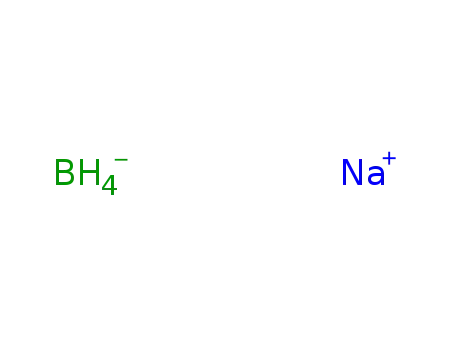
sodium tetrahydroborate

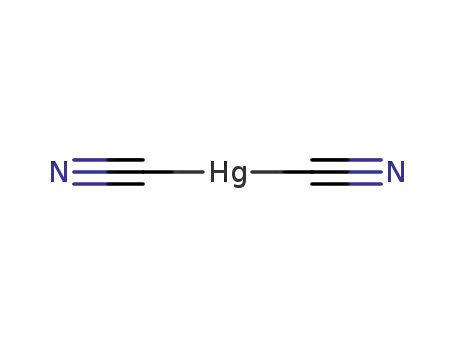
mercury(II) cyanide


sodium cyanoborohydride
| Conditions | Yield |
|---|---|
|
In tetrahydrofuran; byproducts: Hg, H2; molar ratio Hg:B=1:2, refluxing (3 h); sepn. of Hg;
|
78% |

(1S,2R)-1-(3,4-dichlorophenyl)-2-formyl-cyclopropanecarboxylic acid (4-fluorobenzyl)methylamide

(1,3-dioxolan-2-yl-methyl)triphenylphosphonium bromide
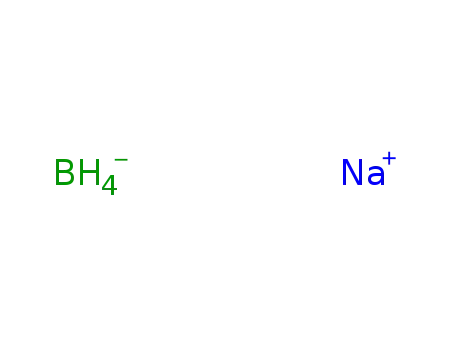
sodium tetrahydroborate

hydrogen cyanide
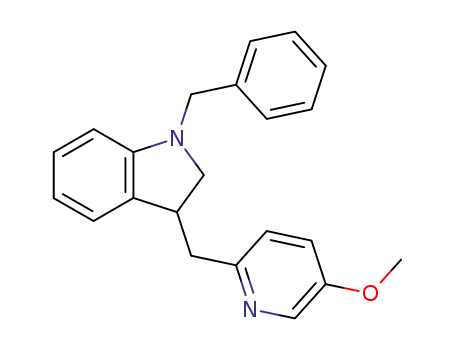
1-Benzyl-3-(5-methoxypyridin-2-ylmethyl)-2,3-dihydro-1H-indol
benzyl-quinolin-2-yl-amine