CasNo: 737789-87-6
MF: C29H27F2N7O5S
|
Description |
Relugolix, is a gonadotropin-releasing hormone antagonist (GnRH receptor antagonist) medication that is used in the treatment of prostate cancer in men and uterine fibroids in women. |
| Use | Relugolix is a GnRH receptor antagonist primarily used in medicine to treat conditions such as prostate cancer, endometriosis, and uterine fibroids by suppressing the production of sex hormones. |
The invention provides a synthesis metho...
The invention provides a preparation met...
The invention provides a novel preparati...
The invention provides a preparation met...
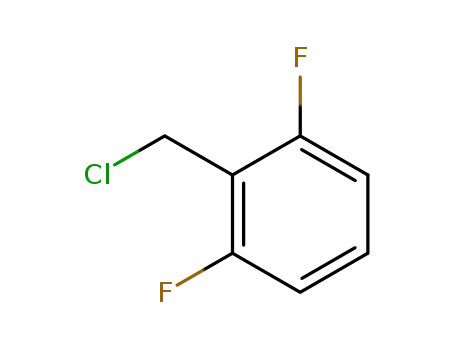
2,6-difluorobenzyl chloride

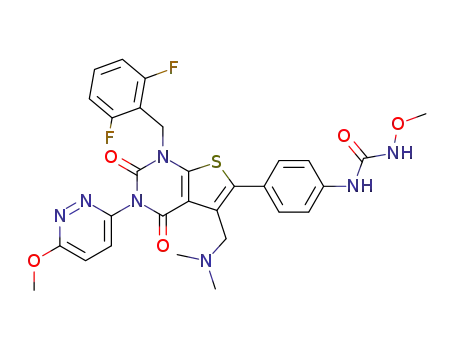
TAK-385
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 7 steps
1.1: N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 5 h / 100 °C
2.1: potassium carbonate / 3 h / 60 °C
3.1: tetrahydrofuran / 5 h / 70 °C
4.1: N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile) / tetrachloromethane / 4 h / Reflux
5.1: potassium carbonate / N,N-dimethyl-formamide / 5 h / 80 °C
6.1: hydrogen; palladium 10% on activated carbon / ethanol / 6 h / 20 °C
7.1: N-ethyl-N,N-diisopropylamine / acetonitrile / 0.5 h / 20 °C
7.2: 4.5 h / 50 °C
With N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile); palladium 10% on activated carbon; hydrogen; potassium carbonate; N-ethyl-N,N-diisopropylamine; In tetrahydrofuran; tetrachloromethane; ethanol; N,N-dimethyl-formamide; acetonitrile;
|
C31H33F2N7O6S


TAK-385
| Conditions | Yield |
|---|---|
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene; In methanol; at 0 ℃;
|
89% |
|
With sodium methylate; In methanol; at 30 - 40 ℃; for 1h;
|
86.3% |
|
With sodium methylate; In methanol; N,N-dimethyl acetamide; at 60 ℃; for 6h; Reagent/catalyst;
|
84.9% |
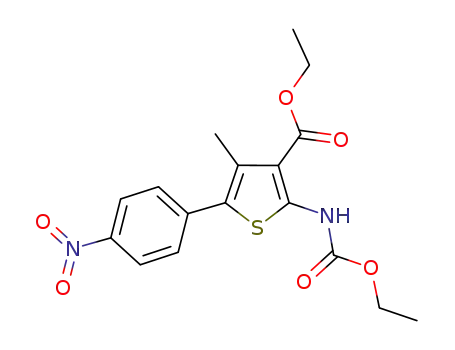
2-((ethoxycarbonyl)amino)-4-methyl-5-(4-nitrophenyl)thiophene-3-carboxylic acid ethyl ester
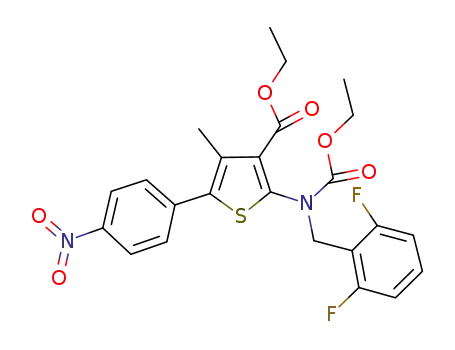
2-((2,6-difluorobenzyl)(ethoxycarbonyl)amino)-4-methyl-5-(4-nitrophenyl)thiophene-3-carboxylic acid ethyl ester
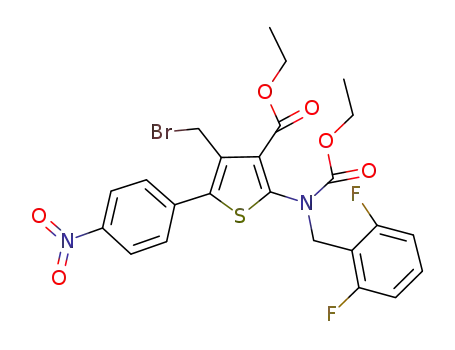
4-(bromomethyl)-2-((2,6-difluorobenzyl)(ethoxycarbonyl)amino)-5-(4-nitrophenyl)thiophene-3-carboxylic acid ethyl ester
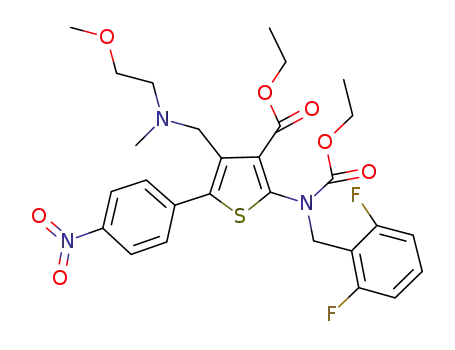
ethyl 2-[(2,6-difluorobenzyl)(ethoxycarbonyl)amino]-4-{[(2-methoxyethyl)(methyl)amino]methyl}-5-(4-nitrophenyl)thiophene-3-carboxylate