Your Location:Home > Products > Inorganic chemistry > trestolone acetate
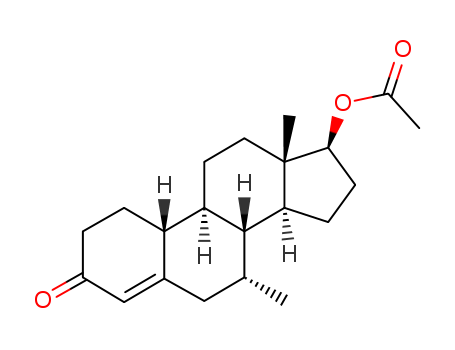

CasNo: 6157-87-5
MF: C21H30O3
|
Side effects |
Trestolone Acetate is quite obvious to offer negative side effects when is considered to be about 10 times more potent than testosterone. Side effects are only possible and they can be well reduced or completely avoided if you know how to use the compound properly.For example, if you would use a PCT plan at the end of the cycle all along with various meds (like Aromatase Inhibitors) and different cycle supporting supplements, without exceeding cycle length and dosage then you would be fine in most cases (unless you have low tolerance or pre existent health issues).Side effects include those of estrogen related (since it aromatizes) and the obvious androgen related ones. All in all, side effects include:AcneHair lossWater retentionHypertensionGynecomastiaAggressionSuppressionNegative effects on lipidsAffects cardiovascular health and othersRemember to use Ment Trest properly if you want to avoid the side effects, because an improper use is almost guaranteeing getting negative side effects. |
|
Definition |
Trestolone acetate is a synthetic and injected anabolic–androgenic steroid (AAS) and a derivative of nandrolone (19-nortestosterone) which was never marketed. It is an androgen ester – specifically, the C17 acetate ester of [DB05830]. |
InChI:InChI=1/C21H30O3/c1-12-10-14-11-15(23)4-5-16(14)17-8-9-21(3)18(20(12)17)6-7-19(21)24-13(2)22/h11-12,16-20H,4-10H2,1-3H3
A series of MENT esters (3-71) was desig...
The use of Cp2ZrMeCl is described as a s...
A series of 3-, 7-, 15-, and 16-methyl-s...
Disclosed is a process for the synthesis...
This invention relates to a process for ...
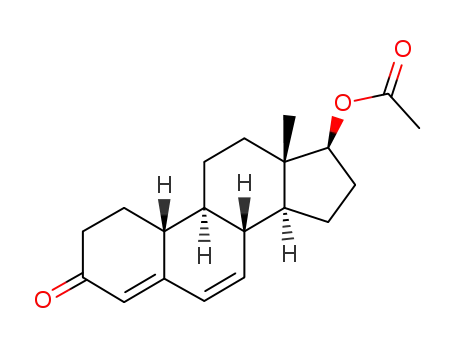
6-dehydro-19-nortestosterone acetate

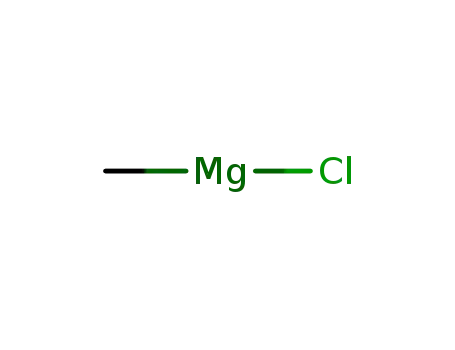
methylmagnesium chloride

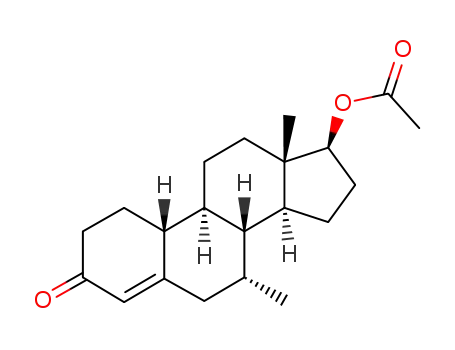
7alpha-Methyl-19-nortestosterone acetate
| Conditions | Yield |
|---|---|
|
6-dehydro-19-nortestosterone acetate; methylmagnesium chloride; copper diacetate; In tetrahydrofuran; at -45 - -35 ℃; for 3h;
With hydrogenchloride; In tetrahydrofuran; water; at 10 ℃; for 0.5h;
|
78% |
|
6-dehydro-19-nortestosterone acetate; methylmagnesium chloride; copper(l) chloride; In tetrahydrofuran; at -30 ℃; for 3 - 4h;
With sulfuric acid; water; In tetrahydrofuran; for 1.33333h;
|

zirconocene methyl chloride


6-dehydro-19-nortestosterone acetate


7alpha-Methyl-19-nortestosterone acetate
| Conditions | Yield |
|---|---|
|
zirconocene methyl chloride; With silver trifluoromethanesulfonate; copper(l) chloride; (3,5-dioxa-4-phospha-cyclohepta[2,1-a;3,4-a']dinaphthalen-4-yl)-bis-(1-phenyl-ethyl)-amine; In diethyl ether; dichloromethane; at 20 ℃; for 0.166667h; Darkness; Inert atmosphere;
6-dehydro-19-nortestosterone acetate; With chloro-trimethyl-silane; In diethyl ether; dichloromethane; at 20 ℃; for 15h; stereoselective reaction; Inert atmosphere; Darkness;
|
61% |
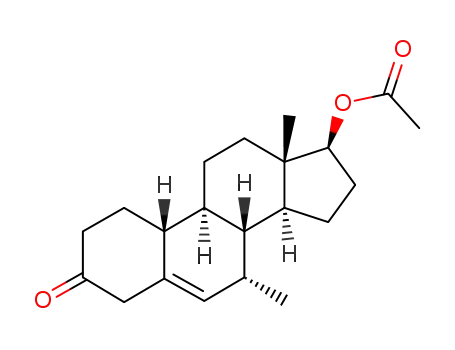
17β-hydroxy-7α-methyl-estr-5-en-3-one acetate
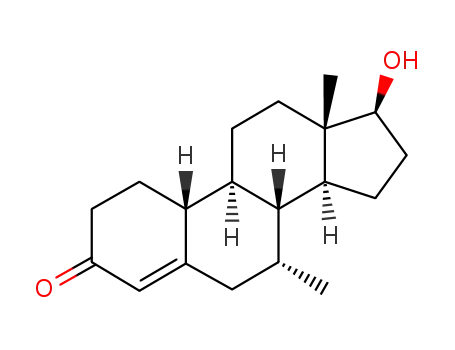
7α-methyl-19-nortestosterone
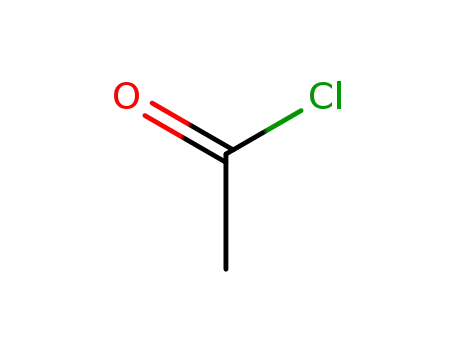
acetyl chloride
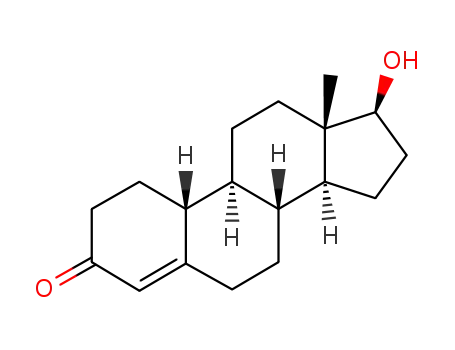
19-nortestosterone
3-methoxy-7α-methylestra-1,3,5(10)-trien-17β-ol

7α-methyl-19-nortestosterone